Calculate the Formal Charge on the Chlorine Cl Atom
So its ah the animal world clock carring the bonnet the bonding No money and the Valance. One electron is claimed by Cl and one by O this means that the chlorine atom owns 7 valence electrons and is thus formally neutral and the oxygen atom also owns 7 valence electrons and thus has a FORMAL negative charge.
Answered Calculate The Formal Charge Of Chlorine Bartleby
Express your answer as an integer.
. Now you have come to the final step and here you have to check the formal charge on phosphorus atom P fluorine atoms F as well as chlorine atoms Cl. View Available Hints formal charge on Cl Submit Part B Calculate the formal charge on each of the oxygen O atoms labeled a b and e in the following Lewis structure. Number of unpaired electrons in Zn2.
So the number of violent and actuals Oh Tom and Manus the some off. Take a pen and paper with you and try to draw this lewis. 7 Express your answers.
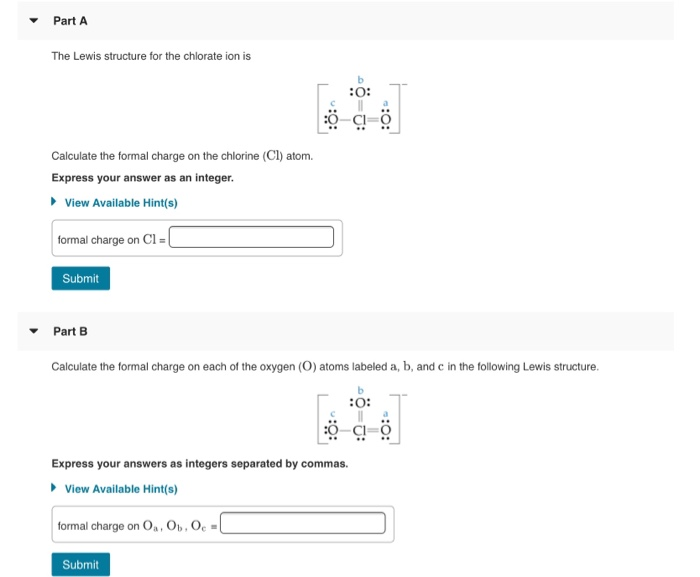
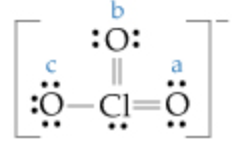
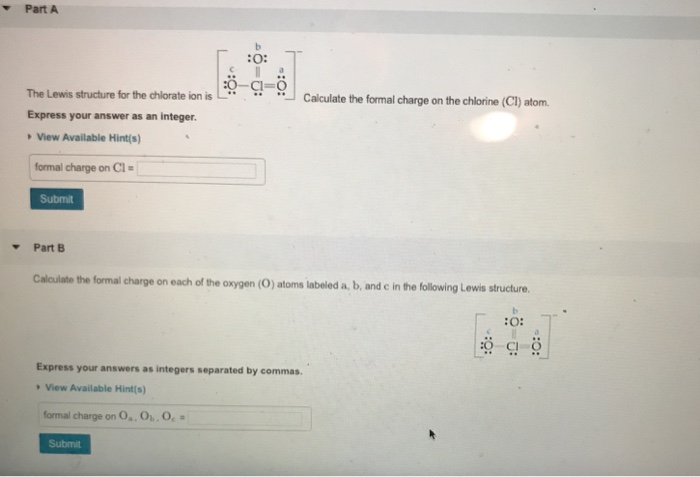
Formal charge on OaOb Oc. All three chlorine atoms have same lone pair and bond pair electrons so we can calculate the formal charge for one chlorine atom also. Part A The Lewis structure for the chlorate ion is O.
Express your answer as an integer. The first look at the chlorine. Hence the formal charge on Cl atom in.
Bonding electrons around central phosphorous atom 8 single 3 bonds with Cl Formal charge on phosphorous 05 0 82 1. Express your answer as an integer. 30-CI Calculate the formal charge on the chlorine Cl atom.
ö-a- Express your answers as. Calculate the formal charge on each of the oxygen O atoms labeled a b and c in the following Lewis structure. Part A The Lewis structure for the chlorate ion is b 0.
Hence the central phosphorous atom of POCl3 lewis structure has 1. In determining the best Lewis structure or predominant resonance structure for a molecule the structure is chosen such that the formal charge on each of the atoms is as close to zero as possible. In order to calculate the formal charges for ClO3- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding e.
Part A The Lewis structure for the chlorate ion is Calculate the formal charge on the chlorine CI atom. The Phosphorus atom P is at the center and it is surrounded by 2 Chlorine atoms Cl. The Phosphorus atom has -1 formal charge.
View Available Hints formal charge on CIEL Submit Part B Calculate the formal charge on each of the oxygen O atoms labeled a b and c in the following Lewis structure. Check the stability of lewis structure by calculating the formal charge on each atom. Calculate the formal charge on the chlorine Cl atom.
Calculate the formal charge on the chlorine Cl atom. For that you need to remember the formula of formal charge. Also calculate the effective nuclear change of N atom.
ScienceChemistryQA LibraryPart A The Lewis structure for the chlorate ion is b 0. So technically the formal charge or red down questions for calculated formal child. Express your answer as an integer.
Express your answers as. Express your answer as an integer. Part A The Lewis structure for the chlorate ion is O.
H C l O 4. Calculate the formal charge on the chlorine Cl atom. That is oxygen Z 8 has 7 valence electrons and 2 inner core electrons and thus 9.
Formal change on Clv-12S-L OR Cl7-128-03 formal change on the three oxygen atom which has an electron pair shared by chlorine is formal charge on 06-122-6-1 Formal change on the oxygen atom which has an electron pair shared by chlorine and another by the hydrogen is formal change on O6-124-0-4 Formal change on the H1-122-00. Because the bonding pair of electron is shared ie. 1 See answer pinky1296 is.
So then its a saying the formal charge. Number of unpaired electrons in Ti2. Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion.
In order to calculate the formal charges for ClO2- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding el. The Phosphorus atom has 2 lone pairs and both the Chlorine atom has 3 lone pairs. 7 0 14 2 0.
Nah boning and burning electrons for this element. What is the formal charge on the chlorine atom in the chlorate ion. View Available Hint s formal charge on CIEL Submit Part B Calculate the formal charge on each of the oxygen O atoms labeled a b and c in the following Lewis structure.
- 9876101 pinky1296 pinky1296 04292018 Chemistry College answered What is the formal charge on the chlorine atom in the chlorate ion. View Available Hints formal charge on Cl Submit Part B Calculate the formal charge on each of the oxygen O atoms labeled a b and c in the following Lewis structure. Calculate the formal charge on the chlorine Cl atom.
7 Express your answers as integers. View Available Hints formal charge on Cl Part B Calculate the formal charge on each of the oxygen O atoms labeled a b and c in the following Lewis structure. Lets draw and understand this lewis dot structure step by step.
The Lewis Structure For The Chlorate Ion Is Calculate The Formal Charge On The Chlorine Cl Atom Wizedu

Solved Part A The Lewis Structure For The Chlorate Ion Is Chegg Com
Solved The Lewis Structure For The Chlorate Ion Is B 0 Chegg Com
Solved Part A O C O Calculate The Formal Charge On The Chegg Com
No comments for "Calculate the Formal Charge on the Chlorine Cl Atom"
Post a Comment